
07
Apr
One Point Zero’s COVID19 Mask Information for Buyers
Since the 20th of January 2020, One Point Zero, with its global teams across Shenzhen China, Hong Kong, Berlin, Zürich, Dublin, London and Paris, have been carefully monitoring the COVID19 situation. Our presence in South China and our close relationship with the Hong Kong government (HK Gov) has led to the setup of a safe, secure and audited supply chain for the HK Gov to procure medical equipment such as respirators, surgical masks, hydro alcoholic gel, disinfectant spray, hospital beds and ventilators.
In addition to managing procurement with the HK Gov medical experts, One Point Zero is in constant communication with advisors from the medical corps in Germany (German military advisors) and France (former APHP director – Assistance Publique Hôpitaux de Paris).
In the following pages, we outline the general purpose information on the articles of first necessity (respirators & masks and how to select them). This is to inform hospital purchasers in Europe, how to select the right masks. This guide is followed by an outlook and conclusion on the state of the market and challenges for the global supply chain.
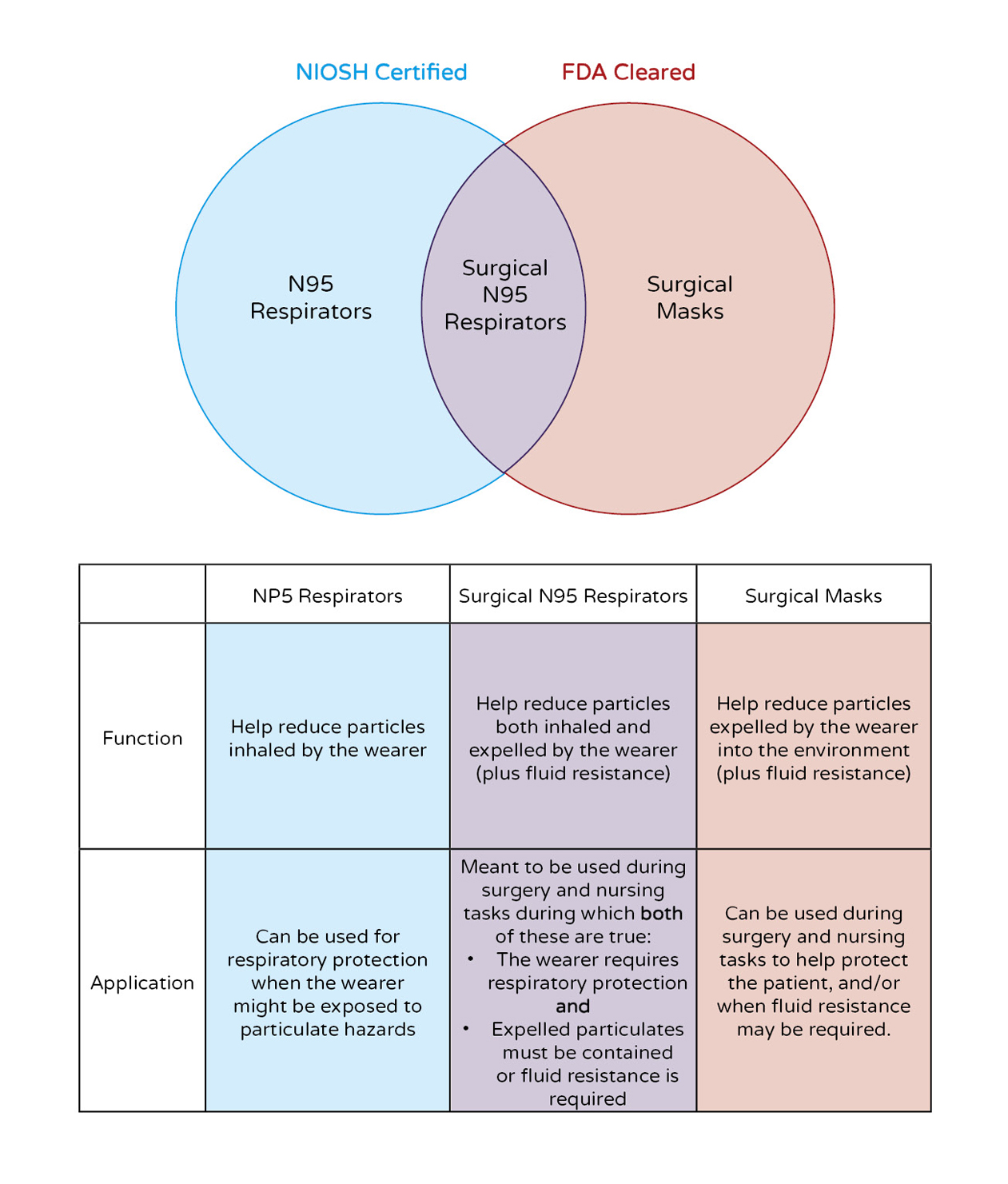
Masks
Masks need to be chosen based on function, fit and filtration capability that matches with the protection needs for each procedure or risk level.
There are 2 key categories:
-
Respirators
-
Surgical masks
The N95 denomination is an American standard and means that the mask Bacterial Filtration Efficiency reaches a minimum of 95%.
CE approved surgical respirators & masks are manufactured by manufacturers that have both:
-
The correct licenses in place, authorizing them to manufacture medical supplies.
-
The correct working standards, in place.
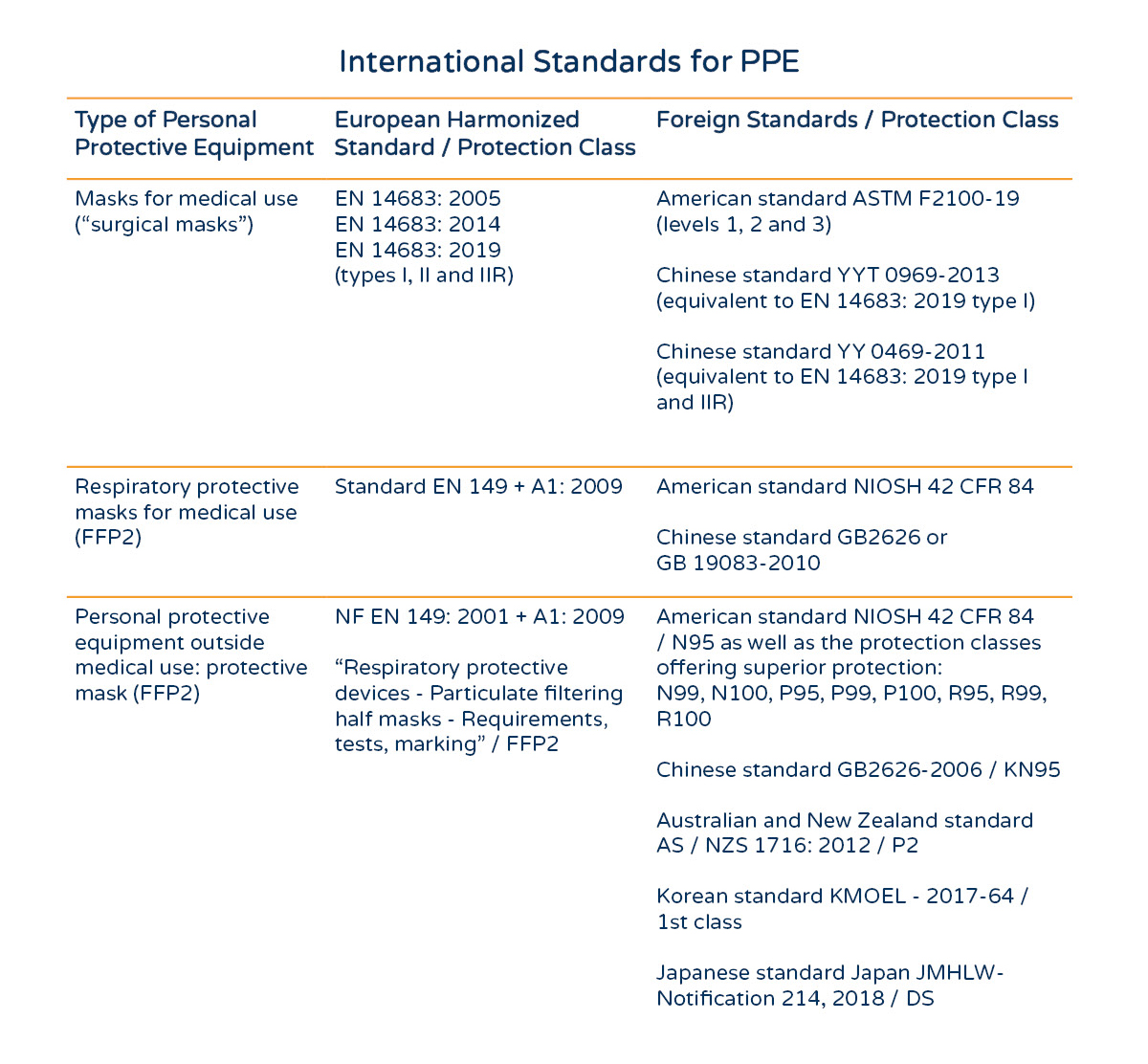
International Standards
China Standards & Correspondence
The Chinese production and certification standard is YY0469-2611
This corresponds to N95 or FFP2 in the EU.
About the masks
-
There are many different types of mask
-
Design & price vary depending on function and intended environment
-
Masks destined for the EU must comply with the following standards: shall follow standard
-
CE2797
-
ISO 149-2001 (respirators, incl. Medical masks)
-
EN14683-2019 (medical mask specific)
-
EU Mask Standards
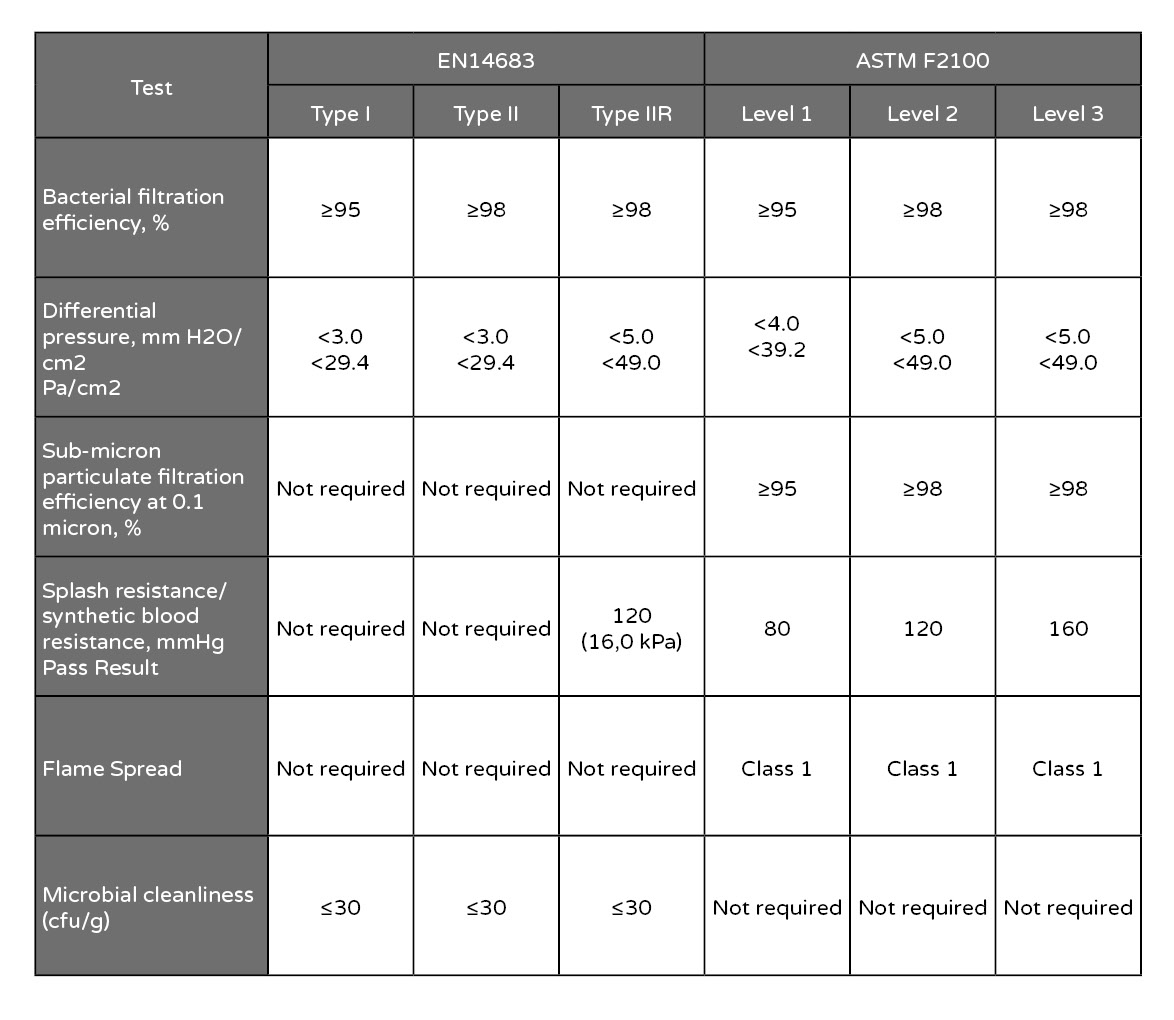
Bacterial filtration efficiency ≥95 = N95 certified equipment.
Key Considerations when Looking for Alternative Suppliers of Masks
Supply & Demand
-
EU hospital & government standard procedures hinder them from being reactive or accessing equipment and protection products fast.
-
Fast financing can be secured by donations from corporations and individuals.
-
Payment terms are 50% at order, 50% at completion of order, before shipment.
> Trust is the number 1 currency and should be established with small orders first
> Masks are the easier items to procure and the most affordable items in the list of response equipment. MOQs start at 20,000 units. Factories have daily capacities of between 200K and 1.5M.
-
There’s no shortage of supply but no stock available. The market is volatile and prices vary every day.
Supply Chain
The issue with the supply chain is the unknown. Although you may not realise it, non-qualified vendors are unable to ship their products as Chinese Customs will seize any goods that do not comply with their regulations. When Chinese Customs approve shipments, there is still a risk that a transit country seize it.

-
The production environment is very unclear. Many consumers’ goods manufacturers are reworking their fabrication lines to build masks, medical supplies and consumables. These new suppliers won’t be ready to deliver goods until they satisfy Chinese authorities’ regulations – as stated above.
-
Certifications procedures alone will take a minimum of a few months before authorization to ship abroad is granted.
Right now, the immediate choice of valid vendors are those that have already met the documentation and qualification requirements.
New manufacturers shall only be considered for a second or third wave of procurement. At that time, many more factories will be able to build and export – risks will have decreased, and market demand will be better managed and directed.
Selecting and working with manufacturers
-
Customers should be careful of purchasing from a new source
-
Ensure third party ISO13485 is valid
-
Business license and export authorization to export medical equipment
-
-
Manufacturers/ inspectors shall perform AQL 4 quality inspections.
-
Manufacturers/ inspectors shall document production and ensure product traceability.
-
Full documentation ensuring traceability shall be released with shipment of goods.
-
One Point Zero has an audited and inspected supply chain already in place.




